Tag: bioequivalence standards
FDA Bioequivalence Standards for NTI Drugs: Special Requirements Explained
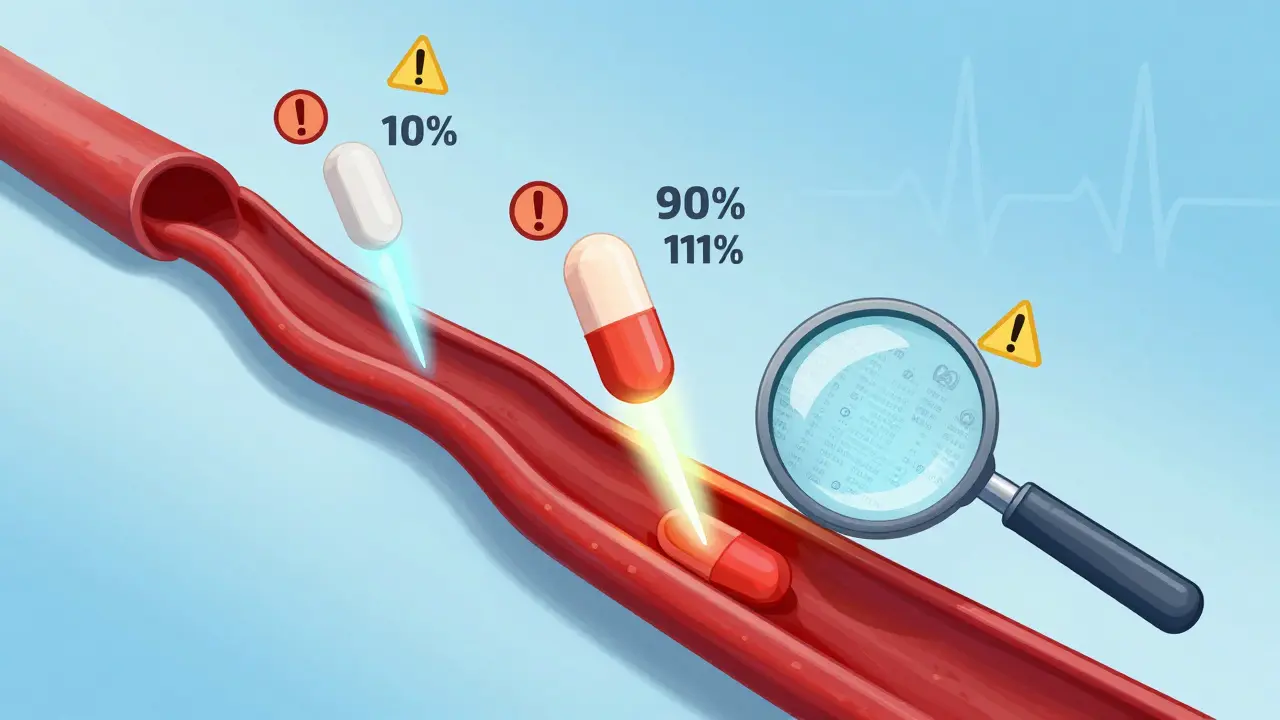
The FDA applies strict bioequivalence rules to narrow therapeutic index (NTI) drugs like warfarin and phenytoin, requiring tighter limits (90-111%) and lower variability than standard generics to ensure safety and efficacy.
Read more