When a doctor prescribes a biosimilar-like Inflectra instead of Remicade-how does the clinic get paid? It’s not as simple as handing in a prescription and getting reimbursed the same way as a generic pill. Biosimilars are complex biologic drugs, not chemical copies. And their billing under Medicare Part B follows a unique, technical system that’s changed dramatically since 2018. If you’re a provider, pharmacist, or even a patient wondering why your out-of-pocket cost varies, understanding this system is key.
How Biosimilars Are Coded Differently from Generics
Unlike small-molecule generics, which all share the same HCPCS code as their brand-name counterpart, each biosimilar gets its own unique code. That’s because biosimilars aren’t identical copies. They’re highly similar, but manufacturing differences mean they can’t be automatically substituted like a generic pill. So CMS assigns each FDA-approved biosimilar its own HCPCS code-either a temporary Q-code or a permanent J-code. For example, Inflectra (infliximab-dyyb) has J1745. Renflexis (infliximab-abda) has J1747. Even though both target the same reference product, Remicade (J1740), they bill under separate codes. This system replaced the old blended model from 2016 to 2017, where all infliximab biosimilars used the same code, Q5101. That created a problem: if one biosimilar launched at a lower price, others could ride on its reimbursement without lowering their own. The 2018 shift fixed that by tying payment directly to each product’s actual price.How Payment Is Calculated
Medicare Part B pays for all biologics and biosimilars at 106% of the product’s Average Selling Price (ASP). That’s 100% of the ASP plus a 6% add-on. But here’s the catch: the 6% add-on is calculated based on the reference product’s ASP, not the biosimilar’s. So if Remicade sells for $2,500 per dose and Inflectra sells for $2,000, here’s what the provider gets:- Remicade: $2,500 + 6% of $2,500 = $2,650
- Inflectra: $2,000 + 6% of $2,500 = $2,150
The JZ Modifier and Discarded Doses
Starting July 1, 2023, providers must use the JZ modifier on claims for infliximab and its biosimilars when no drug is discarded. That means if a vial contains 100 mg and the patient only needs 50 mg, the unused 50 mg is thrown away. If you use the entire vial across multiple patients, you don’t need the modifier. But if you discard any portion, you must report it with the JZ modifier. This rule was added to prevent overbilling. Before, some providers were claiming reimbursement for the full vial even when only half was used. Now, the modifier tells CMS: "No waste here, I used it all." But it’s added paperwork. One gastroenterology practice in Ohio reported a 30% increase in billing staff time just to track vial usage and apply modifiers correctly.
What Happens When a New Biosimilar Enters the Market
The first biosimilar for a reference product gets a special transitional payment. For the first six months after launch, CMS pays 106% of the Wholesale Acquisition Cost (WAC) instead of ASP. That’s because ASP data isn’t available yet-it takes time for sales data to be reported. Once six months of sales data is collected, the payment switches to 106% of the actual ASP. After that, any new biosimilar entering the same market skips the WAC phase. It immediately gets paid based on its own ASP. That’s a big advantage for later entrants-they don’t have to wait for data to build up. But it also means the first mover gets a temporary financial boost, which can be critical for market adoption.Why Adoption Is Still Low in the U.S.
Despite having 32 FDA-approved biosimilars as of late 2023, their market share in the U.S. hovers around 35% for mature products like infliximab. In Europe, it’s 75-85%. Why the gap? The reimbursement structure is a big part of it. In Germany or the UK, biosimilars are often priced through government tendering or reference pricing, where the entire class is reimbursed at the lowest price. That forces providers to choose the cheapest option. In the U.S., the 6% add-on tied to the reference product’s price means providers still make more money with the brand. A 2020 analysis by Dr. Mark Trusheim at MIT found that for every dose of Remicade given, providers earned $30 more than for Inflectra-just from the add-on. That’s not a small incentive. A 2022 survey of 217 cancer centers found that 68% had billing confusion during the 2018 code transition. Even now, 22% of claim denials come from using outdated HCPCS codes. Many practices still use the wrong code because CMS updates its pricing files quarterly, and not all clinics check them.How Providers Can Avoid Billing Errors
Getting paid correctly requires three things:- Use the right HCPCS code for the exact biosimilar administered.
- Apply the JZ modifier when no drug is discarded (for infliximab products).
- Verify the current ASP and payment rate using CMS’s quarterly updates.
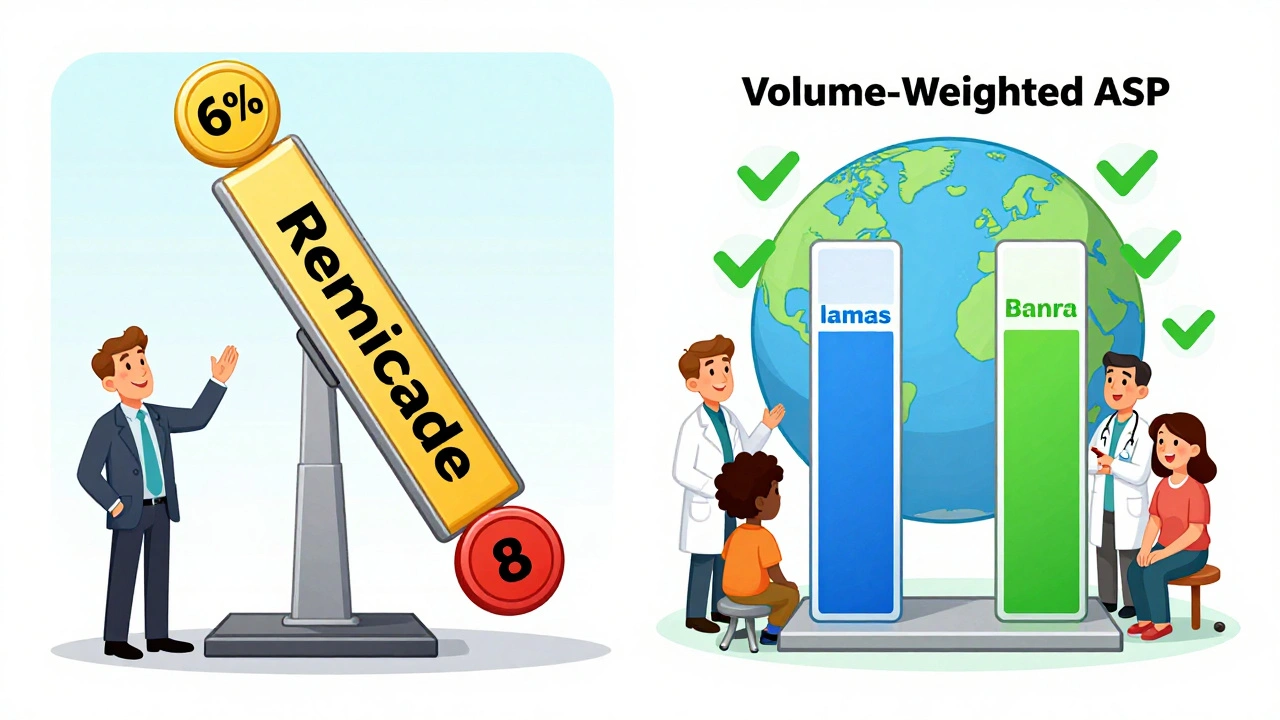
What’s Next for Biosimilar Reimbursement?
CMS is currently reviewing whether to change the 6% add-on structure. One proposal under consideration is to base the add-on only on the biosimilar’s own ASP, not the reference product’s. That would make the financial incentive much stronger. Avalere Health estimates this could increase biosimilar use by 15-20 percentage points across major drug classes. Another idea from MedPAC is "consolidated billing"-paying all biosimilars and the reference product at the same rate: 106% of the volume-weighted average ASP. That’s how many European systems work. It would eliminate the current profit gap and push providers toward the cheapest option. The Congressional Budget Office estimates that if CMS adopted this approach, Medicare could save $3.2 billion over 10 years. But drugmakers warn it could hurt innovation by squeezing profits. It’s a balancing act: lower costs versus keeping biosimilar development alive.What Patients Should Know
Patients don’t control which drug is prescribed, but they can ask. If you’re on a biologic and your doctor suggests switching to a biosimilar, ask: "Will this change my out-of-pocket cost?" Sometimes, Medicare Advantage plans or private insurers have different rules. You might pay less with the biosimilar, but not always-because the 6% add-on doesn’t affect your copay. Your cost-sharing is based on the drug’s list price, not the reimbursement rate. Also, check your Explanation of Benefits. If you see a different code than expected, it could mean your provider switched products without telling you. That’s not illegal, but you deserve to know.Are biosimilars covered the same as brand-name biologics under Medicare Part B?
Yes, but not identically. Both are paid at 106% of their respective Average Selling Prices (ASP). However, the 6% add-on is calculated based on the brand-name product’s ASP, not the biosimilar’s. This means providers earn more per dose when using the brand, even if the biosimilar is cheaper. The patient’s out-of-pocket cost depends on their plan’s copay structure, which may or may not reflect the lower price of the biosimilar.
Why do some biosimilars have Q-codes and others have J-codes?
Q-codes are temporary HCPCS codes assigned to new biosimilars for the first year or two after FDA approval. Once CMS has enough sales data to assign a permanent code, the biosimilar gets a J-code. J-codes are long-term and used for drugs with established market presence. The transition from Q to J doesn’t change payment-it’s just administrative. Providers must update their billing systems when this happens.
Can a pharmacist substitute a biosimilar for the brand-name drug like they do with generics?
No. In the U.S., biosimilars are not considered interchangeable unless the FDA grants that designation. Even then, substitution rules vary by state. Most states require the prescriber to indicate whether substitution is allowed. Without explicit permission, the pharmacy must dispense exactly what’s written on the prescription. This is different from generics, which can be automatically substituted in most cases.
What is the JZ modifier, and when do I need to use it?
The JZ modifier indicates that no portion of the drug was discarded during administration. It’s required for infliximab and its biosimilars under Medicare Part B as of July 1, 2023. If you use the entire vial across one or more patients, you report the JZ modifier. If you discard any amount, you don’t use the modifier. This helps prevent overbilling and ensures payment matches actual usage. Many providers now track vial usage with electronic logs to avoid errors.
How often are biosimilar payment rates updated?
CMS updates biosimilar payment rates quarterly, typically in January, April, July, and October. These updates are based on the most recent Average Selling Price (ASP) data reported by manufacturers. Providers must check the official CMS Physician Fee Schedule updates each quarter to ensure they’re billing with the correct rates. Using outdated codes or prices is a leading cause of claim denials.


Deborah Jacobs
December 5, 2025 AT 23:27Wow, I never realized how much of a mess this system is. I work in a clinic and we still mix up J-codes sometimes - one day we’re billing J1745, the next we’re stuck with Q5101 because the update didn’t sync. It’s like playing whack-a-mole with CMS. And don’t get me started on the JZ modifier. We had to hire a temp just to track vial usage. I mean, we’re doctors, not inventory clerks. 😅
Lucy Kavanagh
December 6, 2025 AT 23:01Of course the Americans can’t do anything right. In the UK, we just pay the lowest price and move on. No fancy modifiers, no 6% add-ons tied to the brand. We don’t let corporations turn healthcare into a profit game. You people let Big Pharma run your system into the ground - and now you’re surprised biosimilars aren’t taking off? Pathetic.
Chris Brown
December 7, 2025 AT 16:58It is, without question, a systemic failure of policy design. The 6% add-on structure is not merely inefficient - it is morally indefensible. By incentivizing providers to favor higher-cost products, the government actively undermines the very principle of cost containment. This is not a technical glitch; it is a deliberate subsidy to monopolistic pricing. The moral hazard is palpable.
Mellissa Landrum
December 9, 2025 AT 01:54sooo… uhhh… this whole thing is a scam right? like, the gov just lets pharma set the price and then pays extra to keep the brand alive?? and the JZ thing?? lol they think we dont know they’re just making us do more paperwork to cover their backs?? my cousin works at a clinic and she says they just fake the logs now. like… why even bother??
Mark Curry
December 10, 2025 AT 17:20It’s funny how we make things so complicated. All we really need is one price for the class. Why does it matter who made it? If it works, use the cheapest. Simple. Like buying apples. No one cares if they’re from Washington or Oregon - just pick the ones on sale. 🤷♂️
Mark Ziegenbein
December 11, 2025 AT 06:45One must consider the broader implications of this reimbursement architecture - it is not merely a fiscal misstep but a profound epistemological failure in the governance of pharmaceutical innovation. The current ASP-based model, tethered to the reference product’s pricing, creates a perverse incentive structure that privileges inertia over progress. The JZ modifier, while administratively burdensome, is a necessary corrective to the moral decay of fee-for-service arbitrage. Yet, the real tragedy lies in the absence of a unified, market-based pricing mechanism - one that recognizes biosimilars not as derivatives but as legitimate competitors. Until CMS embraces a volume-weighted average payment paradigm - as the NHS has done with quiet, unassailable competence - the American system will remain a monument to bureaucratic self-sabotage.
Norene Fulwiler
December 12, 2025 AT 04:05I’ve seen this play out in rural clinics - patients get confused when their copay jumps even though the drug is cheaper. They think the provider’s greedy. But really, it’s the system. I’ve had patients cry because they thought they were being overcharged. We try to explain, but Medicare paperwork doesn’t come with a translation. We need better patient education - not just for docs, but for everyone.
William Chin
December 12, 2025 AT 10:48Per the Centers for Medicare & Medicaid Services, the current reimbursement methodology is in full compliance with Title XVIII of the Social Security Act, Section 1847A. Any assertion that the 6% add-on constitutes an incentive to favor brand-name products is analytically incorrect. The add-on is not a profit margin - it is a statutory payment adjustment designed to cover administrative overhead and ensure access. The burden of compliance rests with providers, not the federal government.
Ada Maklagina
December 12, 2025 AT 18:53so the system’s rigged but we’re all just doing our jobs? cool. i’ll stick with my coffee and ignore the codes.
Harry Nguyen
December 13, 2025 AT 18:30Oh wow, so the government pays providers more to give the expensive drug? And you’re surprised biosimilars aren’t popular? What a shock. Next you’ll tell me people don’t buy soda because it’s healthy. 🤡
Katie Allan
December 15, 2025 AT 03:11This is actually a really thoughtful breakdown. I’ve been working with patients on biosimilars for years and the confusion is real. The good news? More clinics are starting to use those free coding guides from manufacturers - it’s a small win. And honestly, if we can get the add-on tied to the biosimilar’s price, it could change everything. Let’s keep pushing for that.
Michael Dioso
December 15, 2025 AT 13:29You people are clueless. The entire system is designed to keep you dependent. Biosimilars aren’t about cost - they’re about control. The FDA, CMS, and Big Pharma are all in bed together. You think the JZ modifier is about waste? Nah. It’s about tracking who’s using what. They’re building a database. Soon they’ll know your health history better than your doctor. And you’re just sitting there complaining about codes? Wake up.
Krishan Patel
December 16, 2025 AT 04:27Let me be clear - the American healthcare system is a capitalist dystopia disguised as medical progress. In India, we have universal access to biosimilars at 90% lower cost because we do not worship profit. Here, you have a system that rewards greed, incentivizes deception, and punishes innovation with bureaucratic red tape. The JZ modifier? A pathetic Band-Aid on a severed artery. The solution is not incremental reform - it is the complete dismantling of the ASP model and the establishment of a publicly administered pricing regime. Anything less is moral cowardice.